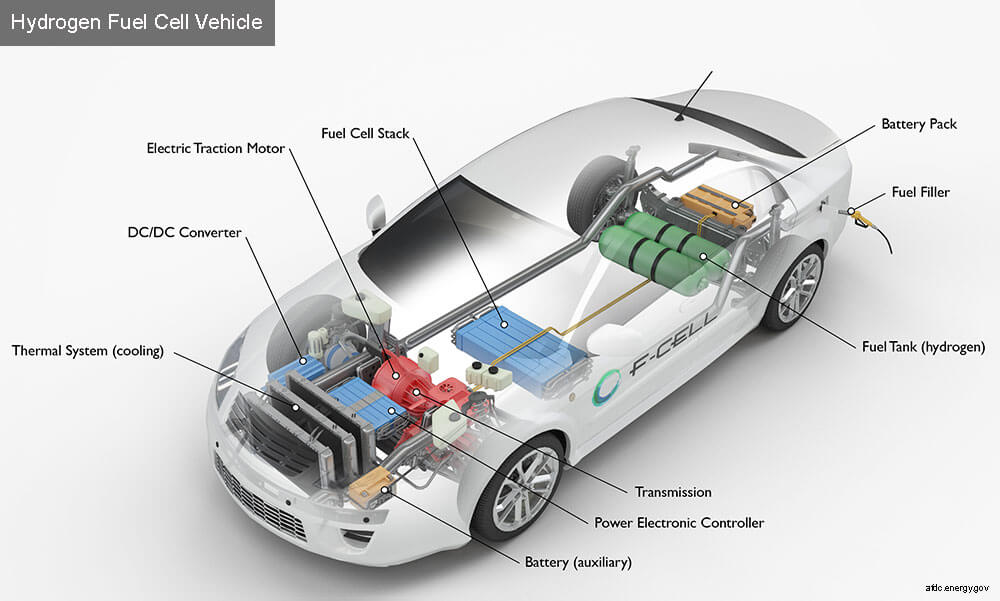
Editor’s note: If fuel-cell EVs ever become a large fraction of the cars on North America roadways, lots of hydrogen will be needed to power them. Ideally, it will be generated by electrolysis and that process will be driven by wind-generated power.
A Florida State University researcher has designed new materials that could be used to store hydrogen fuel more efficiently in vehicles or other devices that use clean energy. Jose Mendoza-Cortes, an assistant professor in the FAMU-FSU College of Engineering, describes his proposed solution and designs for these new materials in an article in the Journal of the American Chemical Society.
“There will be many proposals to solve energy issues, and this may be one option,” Mendoza-Cortes said. “We wanted to find the most effective way to store hydrogen so that perhaps in the future, cars could use this to run longer distances and more efficiently.”
Scientists had already discovered that they needed to pressurize hydrogen to compact it and make it usable as a fuel for cars. But Mendoza-Cortes wanted to take it one step further and make the process more efficient and economically viable.
“We still want to pressurize it, but we want to do it more efficiently,” he said. “Right now, it’s extremely costly to do this.”
Using complex mathematical equations and computer simulations, Mendoza-Cortes designed porous materials of transition metals — compounds involving cobalt, iron or nickel — that cause hydrogen to bond with it. This next-generation design could then be placed in a tank of a car that uses hydrogen for fuel. These new materials are made of Earth abundant elements and therefore are easily available.
Mendoza-Cortes designed 270 compounds through these simulations and then tested their performance for hydrogen storage.
The idea is that since hydrogen will bind to the actual device, more hydrogen could be packed in and condensed into a tank. Because the hydrogen easily sticks to the device, the tank would never actually reach empty. Additionally, he found it would take a smaller energy expenditure to fill up the tank.
“In other words, more hydrogen can be stored at lower pressures and room temperature, making some of these materials good for practical use,” Mendoza-Cortes said.
Currently, hydrogen can be made into liquid at 1 bar — atmospheric pressure — and 20°K or -423.67 Fahrenheit. At that rate, hydrogen can be stored at 71 grams per liter. While at 700 bar and 298°K or 76.73ºF, hydrogen can be stored at 37 grams per liter.
With Mendoza-Cortes’ proposed new materials, hydrogen could be stored at less than 200 bar to fill up the same tank at room temperature, creating a far more efficient system.
“You don’t have to spend all that energy to get the same amount of storage,” he said.
Mendoza-Cortes came to FSU by way of the Energy and Materials Strategic Faculty Hiring Initiative. He is a researcher at FSU’s High-Performance Materials Institute (HPMI), a multidisciplinary research institute dedicated to research and development of advanced materials and manufacturing technologies.
His research is supported by HPMI and Florida State University start-up funds. His postdoctoral researcher Yohanes Pramudya is a co-author on the paper.
Filed Under: Uncategorized