Editor’s note: The electrolyzer discussed below will run on electricity (of course), the cheapest of which is from a curtailed wind turbine. The hydrogen produced provides another way to store excess wind power. Unfortunately, the article omits mention of efficiencies. If the figures that may be forthcoming show an improvement on efficiencies of conventional electrolyzers, such as those from Hydrogenics, then this news is a positive development for wind industry and power users everywhere.
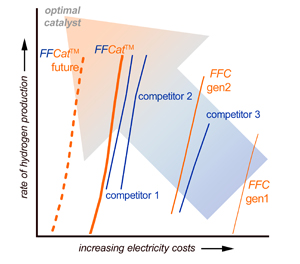
An electrocatalyst is a material that lowers the electrical energy required to drive a reaction. The company says it is developing electrocatalysts to lower the electrical energy necessary to split water (H2O) into hydrogen (H2) and oxygen (O2).
The renewable energy sector is set to undergo a period of unprecedented growth in the coming years, says Bloomberg New Energy Finance. From the group’s 2011 Global Renewable Energy Market Outlook, investment in renewable energy projects is expected to reach $395 billion/year 2020 and $460 bn/yr by 2030. Solar and wind combined will account for $290 bn/yr by 2020. Overall, an estimated $7 trillion in capital will be required from 2011 to 2030.
One challenge facing the deployment of renewable energy systems is the variable nature of renewable energy resources. This drawback presents a huge global opportunity for energy storage systems that can harness excess solar or wind energy and release it during periods where the sun or wind aren’t present. One of the most promising systems is hydrogen (H2) owing to the fact that H2 has a high energy density. One kilogram of H2 holds three times the energy of gasoline (i.e., energy in 1 gallon of gasoline ≡ energy in 1 kilogram of H2). Hydrogen can also power fuel-cell electric vehicles, convert sequestered CO2 into liquid fuels, sold as merchant gas or simply stored until it is needed.
Hydrogen must be extracted from a source because it does not occur naturally alone. The ideal source of H2 is water. FireWater Fuel Corp. says it has developed technology that can produce cheap, scalable, and environmentally friendly hydrogen cleanly from water.
What is the news?
FireWater Fuel says it has developed a line of catalysts for producing hydrogen from water by electrolysis. These new catalysts are based on inexpensive, Earth-abundant materials, including iron oxide.
Water splitting is the separation of water (H2O) into its constituents, hydrogen (H2) and oxygen (O2). However, the process is thermodynamically unfavorable: it requires energy to proceed. Many people may have performed the water-splitting reaction in their high school science class. The inherent problem with this reaction is that it is inefficient. FireWater Fuel says it is focused on the development of electrocatalysts to facilitate water-splitting.
An electrocatalyst is a material that lowers the electrical energy required to drive a reaction. FireWater Fuel says it is developing electrocatalysts to lower the electrical energy necessary to split water (H2O) into hydrogen (H2) and oxygen (O2).
What is an electrolyzer?
An electrolyzer is a device that splits water (H2O) into hydrogen (H2) and oxygen (O2). An electrolyzer is a multi-component system; one key component is an electrode coated with electrocatalysts.
An electrolyzer consumes electricity to generate hydrogen (H2) and oxygen (O2) from water (H2O). A fuel-cell performs the reverse reaction, combining hydrogen (H2) and oxygen (O2) to produce electricity. The FireWater Fuel team says it is currently developing a prototype electrolyzer.
FireWater Fuel Co.
www.fwfuel.com
Filed Under: Energy storage, News





If one understands what a photon really is you should be able to come up with a solution to creat a catalyst to separate hydrogen with electrolysis.