A reduction-oxidation (redox) flow battery is an electrochemical storage device which stores energy in a chemical form. Redox, a contraction of reduction (a gain of electrons) and oxidation (a loss of electrons), is a chemical reaction in which electrons are transferred between chemical species. Using the same element on both sides eliminates cross-contamination issues. The stored chemical energy is converted to an electrical form via spontaneous reverse redox reactions. To restore the dispensed chemical energy, an electrical current is applied to induce the reverse redox reaction.
Hybrid flow batteries deposit one or more of the electro-active materials as a solid layer on an electrode. Hybrid flow batteries include a chemical that forms a solid precipitate plate on a substrate at a point throughout the charge reaction which may also be dissolved throughout the discharge reaction. During the charge reaction, the chemical may solidify on the surface of the substrate forming a plate near the electrode surface. The chemical is regularly a metallic compound.
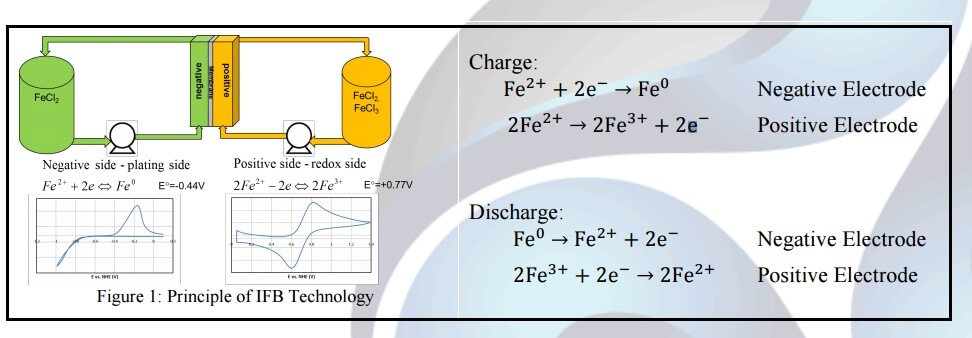
In hybrid flow battery systems, the energy stored by the redox battery may be limited by the amount of metal plated during charge and may accordingly be determined by the efficiency of the plating system as well as the available volume and surface area to plate. Unlike typical batteries that are packaged as fixed cells or modules, a flow battery allows the battery’s power (the rate of electricity flow) to be decoupled from the battery’s capacity (the total amount of energy held). As a result, users are free to tune the battery’s specifications to their specific needs. One example of a hybrid redox flow battery is the all-iron redox flow battery (IFB) developed by ESS.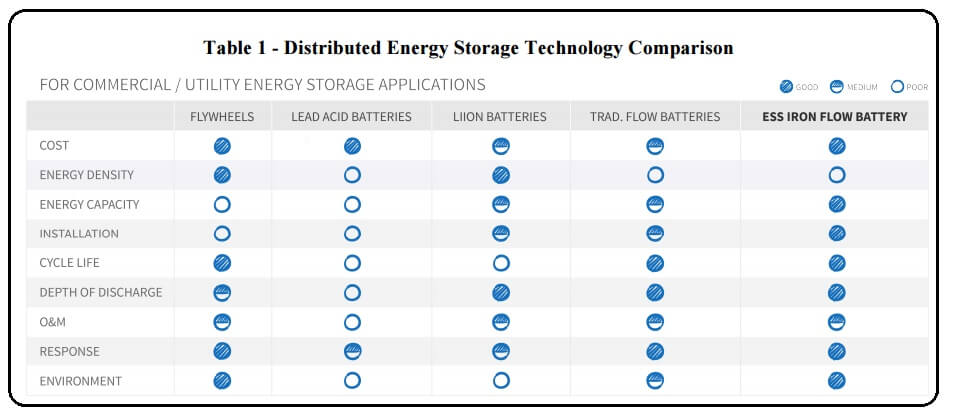
The IFB technology uses iron as an electrolyte for reactions including a negative electrode where plating occurs, herein also referred to as the plating electrode, and a positive electrode where a redox reaction occurs, herein also referred to as the redox electrode. The performance of an IFB battery can be broken down to its plating electrode performance (negative electrode), redox electrode performance (positive electrode), and ohmic resistance loss. On the plating electrode, the ferrous (Fe2+) ion gains electrons and plates as solid iron on the substrates during charge, as shown in Figure 1 below, and the solid iron dissolves as ferrous ions and releases two electrons during discharge. The equilibrium potential for the iron plating reaction is -0.44V. On the redox electrode, the redox reaction between ferrous and ferric (Fe3+) ions occurs during charge and discharge. On the positive electrode, two Fe2+ ions lose two electrons to form Fe3+ ions during charge and two Fe3+ ions gain two electrons to form Fe2+ during discharge. The equilibrium potential between ferrous and ferric ions is +0.77V. Thus, the reaction in an IFB redox flow battery is reversible.
The ESS team has innovated upon this simple yet elegant electrochemistry and enabled this traditional IFB technology to operate much more efficiently (US20140065460). Our patented battery design combines plentiful and extremely cost effective materials with an innovative cell design that dramatically increases power density and enables a smaller, less costly power stack. ESS IFB users can expect over 10,000 cycles at over 80% depth of discharge during a 25-year life, with minimal maintenance. ESS has cracked the code to keeping traditional iron chemistry stable for thousands of deep charge and discharge cycles with no degradation. ESS’ patent-pending electrode designs allow you to operate at high flow-battery efficiency levels (US20140272493, US20140363747, US20150255824). In addition, ESS has scaled up and validated its unparalleled battery technology from Watts to Multi-kW power modules (Figure 2) and from a single power module to fully-integrated, multi-power module, turnkey systems (Figure 3).
Filed Under: Energy storage



I was not able to download this white paper — the URL was not found.
I am extremely puzzled by the electrochemical scheme shown here. Since Iron(0) is insoluble, how is it transported to and from the electrodes? How is this device truly a “flow” battery?